Packaging Symbols
Symbols used on the packaging and labelling of GBUK products. All GBUK products are marked with internationally recognised and approved symbols. These indicate key information about manufacturing and product expiry dates, standards compliance and optimum storage conditions.
| Title of symbol | |
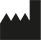 |
Manufacturer |
 |
Country of manufacture |
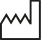 |
Date of manufacture |
 |
Use-by date |
 |
Authorized representative in the European Community |
 |
Batch code |
 |
Catalogue number |
 |
Serial number |
 |
Medical device |
 |
Unique device identifier |
 |
Sterile |
 |
Sterilized using aseptic processing techniques |
 |
Sterilized using ethylene oxide |
 |
Sterilized using irradiation |
 |
Sterilized using steam or dry heat |
 |
Sterilized using vaporized hydrogen peroxide |
 |
In vitro diagnostic medical device |
 |
Control |
 |
Negative control |
 |
Positive control |
 |
Single sterile barrier system |
 |
Double sterile barrier system |
 |
Single sterile barrier system with protective packaging inside |
 |
Single sterile barrier system with protective packaging outside |
 |
Do not resterilize |
 |
Non-sterile |
 |
Do not use if package is damaged |
 |
Sterile fluid path |
 |
Fragile, handle with care |
 |
Keep away from sunlight |
 |
Protect from heat and radioactive sources |
 |
Keep dry |
 |
Importer |
 |
Distributor |
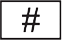 |
Model number |
 |
Brakes on |
 |
Buckle patient |
 |
Centre patient |
 |
Do not wash |
 |
No latex |
 |
Max weight |
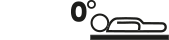 |
Patient flat |
 |
Refer to manual |
 |
Two caregivers |
| Title of symbol | |
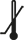 |
Lower limit of temperature |
 |
Upper limit of temperature |
 |
Temperature limit |
 |
Contains human blood or plasma derivatives |
 |
Contains a medicinal substance |
 |
Contains biological material of animal origin |
 |
Contains biological material of human origin |
 |
Contains hazardous substances |
 |
Contains nano materials |
 |
Humidity limitation |
 |
Atmospheric pressure limitation |
 |
Biological risks |
 |
Do not re-use |
 |
Consult instructions for use |
 |
Caution |
 |
Contains or presence of natural rubber latex |
 |
Contains sufficient for ‘n’ tests |
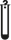 |
For IVD performance evaluation only |
 |
Sampling site |
 |
Fluid path |
 |
Non-pyrogenic |
 |
Drops per millilitre |
 |
Liquid filter with pore size |
 |
One-way valve |
 |
Patient number |
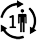 |
Single patient – multiple use |
 |
Patient name |
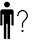 |
Patient identification |
 |
Patient information website |
 |
Health care centre or doctor |
 |
Date |
 |
Translation |
 |
Repackaging |




